Apheresis Device

The Adacolumn medical device
For therapeutic Leukocytapheresis
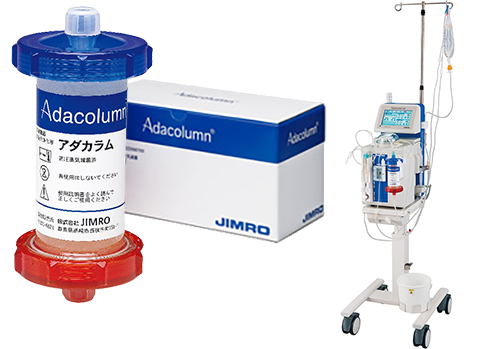
This is a picture of the Adacolumn, which is being used in Japan.
The Adacolumn is a medical device used in clinical practice setting for the treatment of patients with active inflammatory bowel disease, mainly ulcerative colitis and Crohn’s disease. Additional indications for the Adacolumn are listed below and include patients with pustular psoriasis, psoriatic arthritis. The mechanisms of action of the Adacolumn are adsorptive depletion of elevated and activated myeloid lineage leukocytes primarily granulocytes and monocytes from peripheral blood through extracorporeal circulation.
The Adacolumn is CE certified, “Conformite Europeenne” for the flowing conditions.
Ulcerative colitis, Crohn’s disease, Rheumatoid arthritis*, Behcet’s disease*, Systemic lupus erythematosus*, and pustular psoriasis.
*indicates currently it is not approved for clinical use in Japan.
Product Inquiry
In Europe, JIMRO has granted exclusive sales rights to Adacyte
Therapeutics SL. Accordingly, within Europe, all inquiries related to
the Adacolumn should be directed to the Adacyte, or access the company’s
website: Adacyte Therapeutics SL